

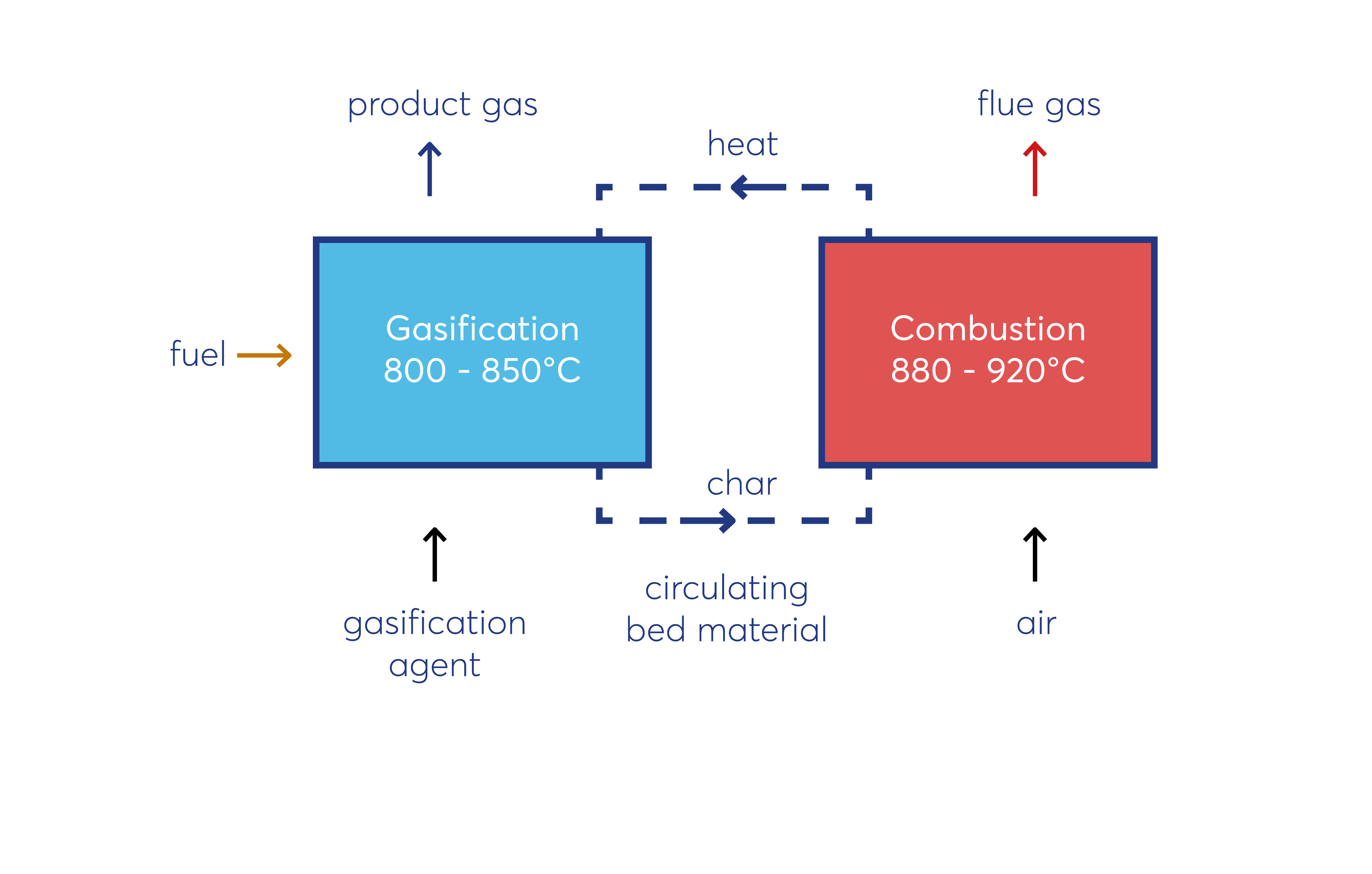
Gasification Technology
The gasification technology provides a thermo–chemical conversion of solid or liquid fuel into gaseous fuel by reaction with a gasification agent. Main driving force for the conversion process is the presence of heat. A complete combustion, a full oxidation, of the fuel particle is prevented because of a lack of available oxygen. As gasification agent oxygen (O2), steam (H2O), carbon dioxide (CO2) and hydrogen (H2) can be used. For practical applications, air, steam or steam - oxygen mixtures are used to gasify various fuels.
The conversion of the biogenic fuel particle goes through different stages. The basic principle of gasification is depicted in the subsequent
figure.
At the first stage (Drying) the wet particle gets dried and hot steam volatilizes. At the second stage (Pyrolytic decomposition), the dried particle gets decomposed by heat into char and gaseous products of pyrolysis
(Volatiles). At the final stage (Gasification) the char gets gasified in the presence of a gasification agent. This leads to a release of char gasification products and ash is produced. Gaseous products of each single stage at the end
react with each other in homogenous reactions to the product gas.
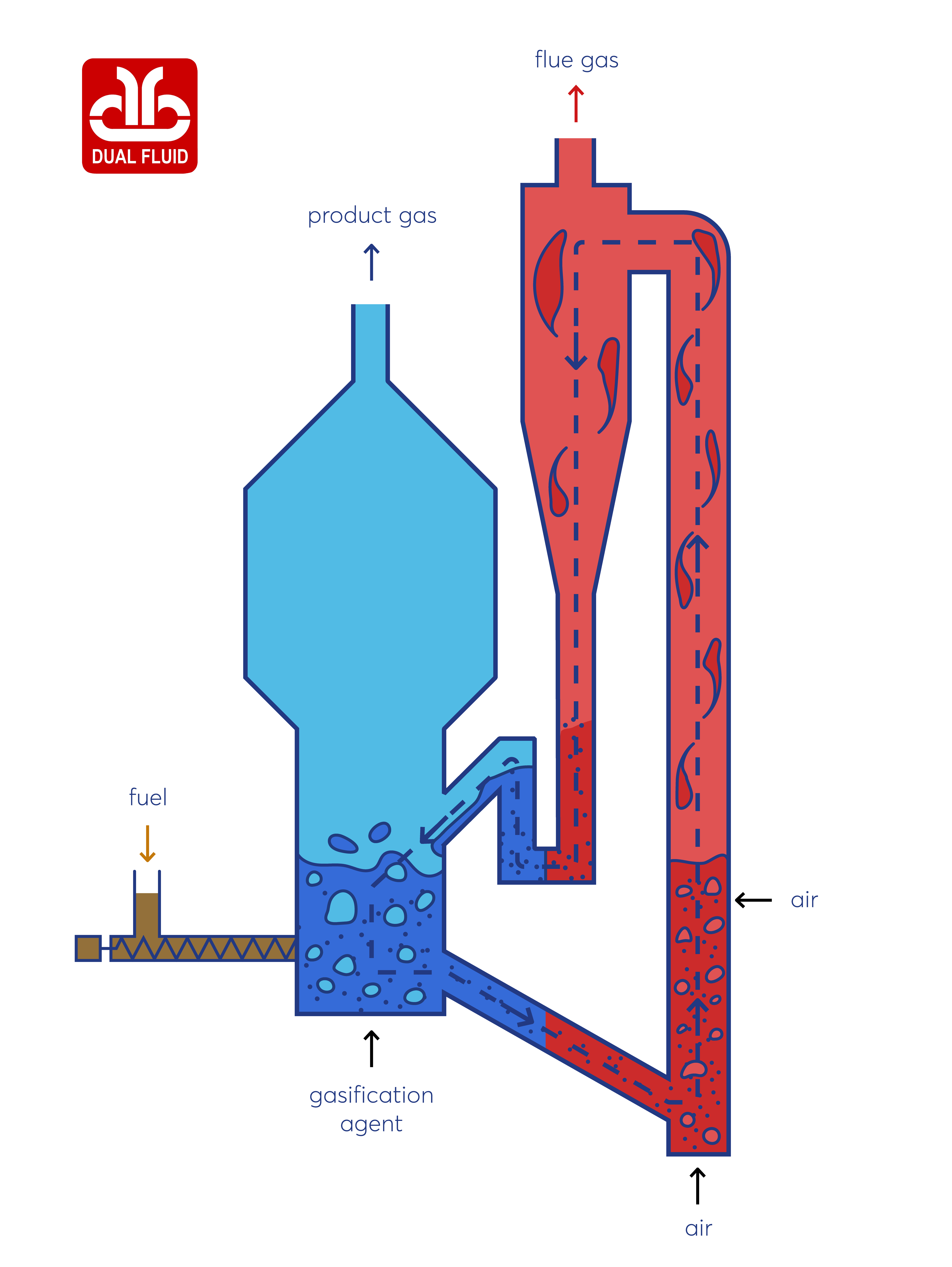
Combined Heat and Power
Producing power and utilizing available heat in a combined heat and power plant is one of the most efficient ways to cover globally rising energy demands. Explore the combined heat and power solutions by VERTO and learn how you can achieve your energy goals at lower costs while keeping emissions at a minimum. VERTO provides services for the innovative Dual Fluid (official trademark of VERTO) gasification technology which offers the opportunity to use a big variety of biogenic based fuels. The gasification with steam provides a medium calorific product gas with high amounts of hydrogen (H2) and carbon monoxide (CO). These properties make the product gas available for numerous utilization possibilities.
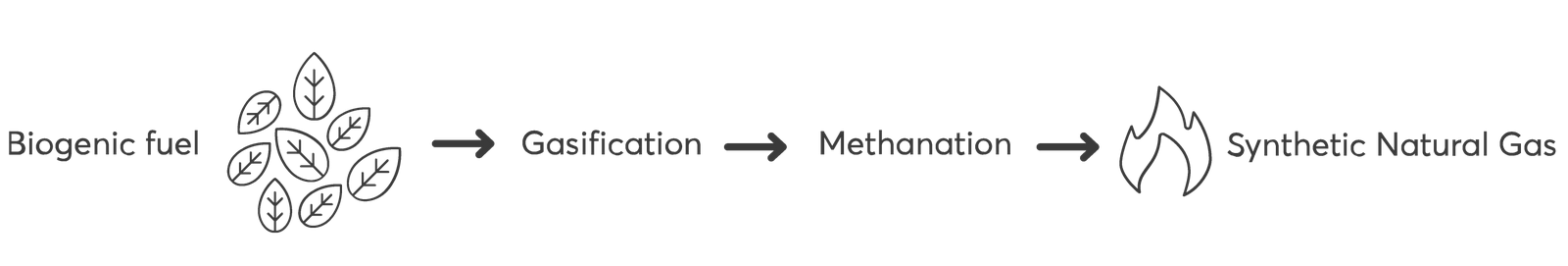
Synthetic Natural Gas
The innovative VERTO dual fluid bed steam gasification process provides a favorable product gas composition with high contents of hydrogen (H2) and carbon monoxide (CO), and no dilution with nitrogen (N2). Therefore, the product gas is very suitable to feed a methanation process. A methanation process aims to increase the methane (CH4) content of the processed gas so that the feed-in conditions in a natural gas grid can be met. The produced synthetic natural gas can supply a large sales market with a subsidized selling price.


BiofuelS
Biofuels are an alternative to fossil fuels as a renewable energy dense carbon source and are a key technology for replacing petroleum products such as gasoline, diesel, and kerosene. Furthermore, biomass is the only renewable carbon source. The reduction of emissions and the promotion of sustainable energy sources are core issues of modern energy policies. Biofuel can be synthesized among others out of synthesis gas for example by the Fischer-Tropsch process. The synthesis gas can be obtained from a broad spectrum of biogenic materials through gasification.
Hydrogen
Hydrogen is used as an important feedstock for the chemical industry. It is used for the production of ammonia, hydrotreating of fossil fuels, as a reducing agent for iron production, as well as fuel for hydrogen powered cars. Most of the hydrogen is currently produced from fossil fuels like natural gas, causing significant fossil CO2 emissions. Alternatively, hydrogen can be obtained from renewable sources without affecting the characteristics of its products and can therefore be integrated as part of a sustainable energy strategy.
Hydrogen from biomass
Hydrogen can be obtained from biomass by gasification in combination with the water gas shift reaction and several gas cleaning steps such as gas scrubber or pressure swing adsorption to produce hydrogen with very high purity. The recovered
hydrogen is a valuable product with a broad spectrum of applications like an energy source, reagent for various syntheses or reducing agent for ore reduction.

Fluidized Bed Ore Reduction
An ore reduction process can be efficiently realized by the aid of a fluidized bed. The fluidized bed offers optimal conditions for an excellent gas-solid interaction and heat transfer. The ideal particle size is between 0.02 mm and 2 mm so that a fluidized bed will form. Under the presence of a reduction agent like hydrogen (H2) or carbon monoxide (CO) iron oxides like hematite (Fe2O3), magnetite (Fe3O4) and wustite (FeO) can be efficiently reduced to raw iron (Fe). Based on the fluidized-bed principle of iron reduction a novel process concept has been developed, the VERTORED process. The VERTORED process uses natural gas or (brown) coal to provide the necessary reduction agent and the process heat.
